The process of passing a small electrical current through a specialised cell in a solution of tap water (H20) and salt (NaCL) is called electrochemical activation (ECA).
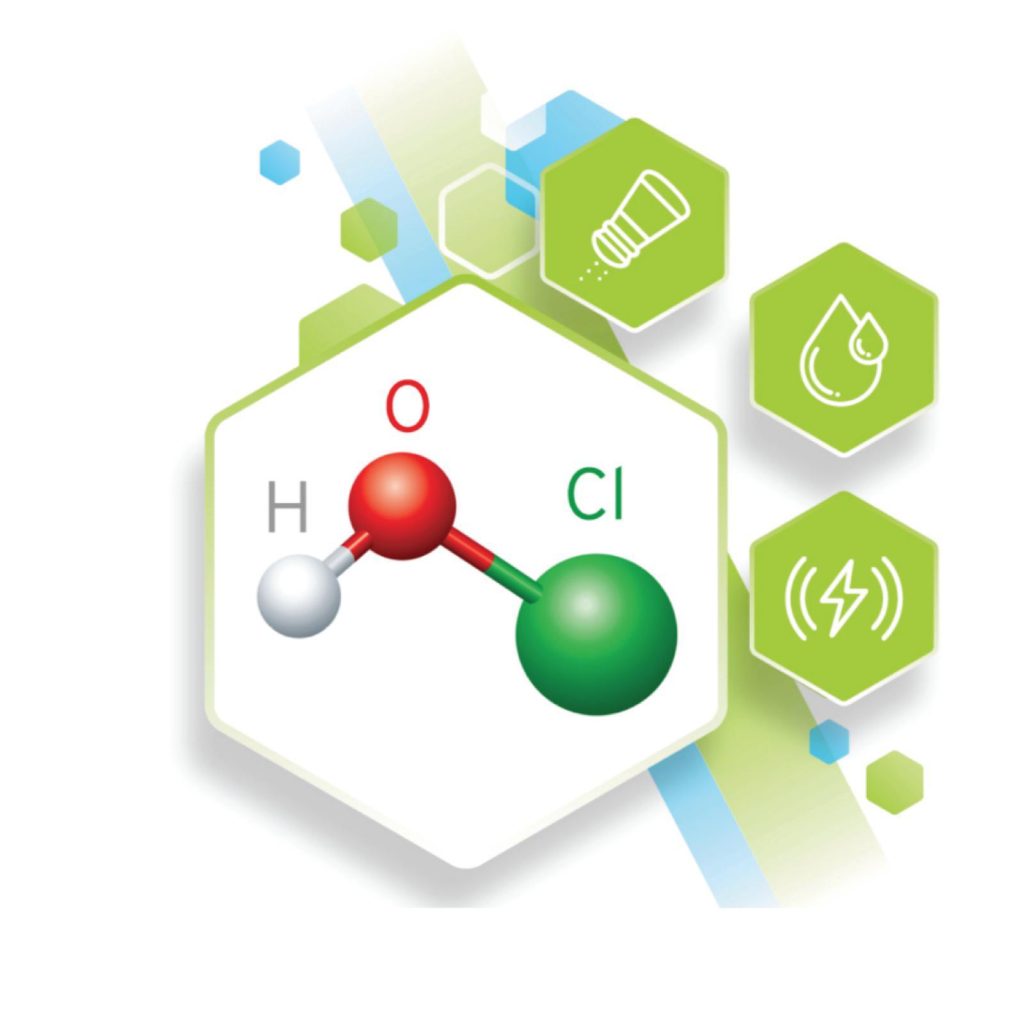
This allows the hydrogen and oxygen in the water to react with the chlorine in the salt to produce ‘free available chlorine’ (FAC), of which the key active substance is Hypochlorous Acid (HC10), this is a powerful but safe disinfectant. The sodium in the salt combines with the hydrogen and oxygen in the water and small amounts of chloride in the salt to produce a mild form of sodium hypochlorite. This is a good cleaning agent. The activation process takes minutes depending on the volume of solution required
The performance of a disinfectant is measured by its concentration – parts per million (ppm) of the active substance – in this case FAC. Many microorganisms can be destroyed with a concentration of around 2Oppm of FAC. In fact, drinking water contains free available chlorine levels of between 0.2 — 2ppm E-storm devices can make concentrations of around 65 to 100ppm FAC. Of this, around 20% is in the form of Hypochlorous Acid. which has a very high efficacy in eliminating microorganisms and represents the optimum level for cleaning and disinfection maintenance.
Another measure – three in fact – is called kill kinetics’, developed to measure the three stages of an effective disinfectant. These three measures consist of the kill rate or efficacy (the percentage of bacteria killed), the speed of the kill (contact time) and the rate of any regrowth Let’s use an example and take the kill rate first. If we took a bacterial count (Colony Forming Units or CFUs) of one million on a given surface and applied disinfectant rated with a killing power of 99.9%, then 99.9% of the CFUs would be killed leaving 1,000 remaining. If a disinfectant was used with a killing power of 99.999% then only 10 of the bugs would be left alive out of the original one million. Quite effective, don’t you agree? British Standards when testing E-storm solution proved that it has excellent bacterial killing performance, carrying the EN1276 accreditation.
HOCl is used in wound care, skin disinfection, food safety, water treatment, purification of oil and natural gas wells, deodorization and skin products, and as a food-safe sanitizer on both food products and food preparation surfaces.
It is a strong disinfectant in health care and is an excellent cleaning solution.

Contact time and rate of regrowth
The remaining two ‘kill kinetics’ are contact time and rate of regrowth. British Standards also prove that the solution kill rate is less 5 mins which is not always the case with disinfectant with some taking five, ten, fifteen or even thirty minutes. And how quickly do any surviving microorganisms recover and start to multiply after the solution has been applied? In trials, after 24 hours there wasn’t any evidence of regrowth with E-Storm.
We’ve mentioned that the solution cleans as well. While the activation of the.disinfectant is taking place, the sodium and chlorine in the salt is combining with the oxygen in the water to create sodium hypochlorite (Nacl0); a very mild form of bleach. This compound gives the solution its cleaning properties with a pH level of between 5 – 6.5. This allows the solution to be used in most areas that would normally be used by conventional mass-produced cleaning chemicals.
Technology inspired by our very own immune system
When the body comes under attack from invading bacteria and viruses, the immune system responds by sending white blood cells called neutrophils straight to the infection Once activated, these cells produce large amounts of Hypochlorous Acid (HOCI) to eliminate invading microbes and pathogens. This is amongst the most potent and naturally occurring disinfectants.
E-Storm technology creates the same HOCI solution, but in a more concentrated form. This means the solution does not give any reaction in the form of irritation, breathing difficulties or allergies when it comes into contact with the skin, eyes, or mouth, making the solution totally safe to use around babies, children, the elderly, and people with compromised immune systems or allergies. Even if ingested there are no harmful effects.
HOCL has a History
In 1834, the French chemist Antoine Jerome Balard discovered Hypochlorous Acid (HOCl) when he added a dilute mix of mercury (II) oxide in water to chlorine gas. Additionally, he discovered that HOCl was an effective and safe disinfectant solution.
Later in the 19th century, Michael Faraday was the first scientist to develop a successful technique that generated HOCl from saltwater. The process was called and continues to be known as electrochemical activation.
Early technology posed significant challenges in producing pure and stable Hypochlorous Acid, making it nearly impossible. In the past, manufacturing HOCI was costly and most products lacked the necessary stability and shelf-life for extended usage. However, modern technological advancements have revolutionized the production of HOCI, unlocking its natural potential and enabling us to utilize it freely as required.
Scientific research now extensively backs the utilization of Hypochlorous Acid in various applications. Numerous studies have been conducted and published, highlighting the efficacy of HOCI in both dermatological and surgical fields. The findings present convincing evidence that HOCI is a practical choice for wound management. Additionally, research supports the application of topical Hypochlorous Acid for treating a range of inflammatory skin conditions such as atopic dermatitis and acne vulgaris.
Coupled with its applications in industries like food processing, veterinary care, and hospitality as a disinfectant and cleaner, the versatility of Hypochlorous Acid makes it a potent substitute for traditional chemicals in shaping our future.

Now you can clean & kill 99.9% of germs without toxic chemicals. BS - approved. Sign up to get 10% off.